The kimoto method creates a depth of flavor that modern sake-making methods simply cannot match.
We believe that this distinction in production methods is more profound than merely the difference in rice polishing ratios.
The kimoto method consists of numerous complex processes.
What is the kimoto method
〜images showing
the 3 main processes〜
The kimoto method was established around the year 1700 and is the most traditional and orthodox way to brew sake. The process of first cultivating yeast in a small tank before the fermentation in the large tank takes place is called moto or shubo. In the case of the kimoto method, we make use of the struggle for survival of natural microbes, a complex process for which high skill is necessary and which takes three times longer than normal. The result is an excellent yeast, very pure and powerful. Below you can see images of the 3 most important steps, “preparation,” “mash grinding” and “applying mash warmers.”
Preparation (shikomi)
The process of cultivating a concentration of superb yeast is called moto and the most orthodox way of doing this is kimoto.
The kimoto preparation (shikomi) involves first putting a certain volume of moto-koji, steamed rice and brewing water into hangiri tubs, which are wide and shallow. Eight hangiri tubs are prepared at one time and the contents are mixed well.
Mash grinding
(motosuri or yama-oroshi)
The mash grinding process is unique to the kimoto brewing method. In Japanese it is known as motosuri or yamaoroshi.
By using a tool called a kaburagai, the steamed rice and koji are carefully ground into a paste.
The process is repeated three times for each of the eight hangiri tubs.
(Mash Grinding Song by the contemporary Eishotai)
Applying mash warmers (daki-ire)
Applying mash warmers (daki-ire) is the step where hot water containers called daki-daru are used to raise the temperature and increase microbial activity. The carefully ground mash from the eight hangiri tubs is collected into one small tank called tsubodai. The temperature is gradually raised by using mash warmers. Lactic acid bacteria grow, producing lactic acid that eliminates other bacteria. Yeast is added at this point and the strong yeast multiplies prolifically. Eventually so much heat is produced by the yeast itself that the mash warmers are no longer needed.
The kimoto method,
a microbial mystery
The microbial drama
of the kimoto tanks
The eve of battle
Steamed rice, koji and water are all mixed in the mash tank. In the early low-temperature stages, the mash is nutrient-poor and the situation is ripe for warring factions of microbes. Quickly gaining strength among the rival groups are the nitrate-reducing bacteria. They enter from the brewing water and rice, and by oxidizing the nitrate salts in the water to nitrous acid they attack the other microbes present. However the film yeast and wild yeast that have infiltrated the tank as yet sustain no decisive damage.
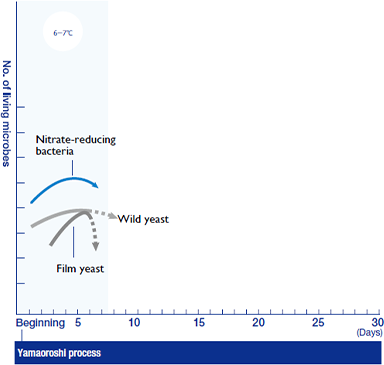
Lactic acid conquers all
As the temperature rises, the koji little by little begins to produce nutrients. This allows the lactic acid bacteria, which have entered from the air and the koji, to become active. They begin to produce lactic acid from the glucose. Most microbes dislike the acidic environment created by lactic acid. The initially powerful nitrate-reducing bacteria are wiped out. Even the tough film yeast and wild yeast are vanquished by the double-barreled attack of nitrous acid and lactic acid. Thus, the lactic acid bacteria conquer all to reign supreme within the mash tank, thanks to the acid they produce.
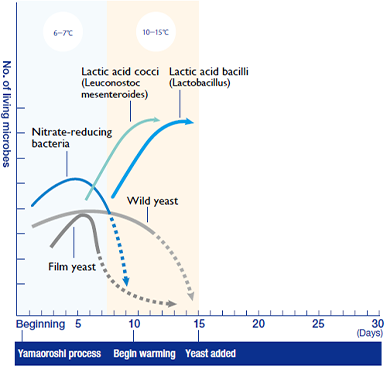
And then there were none
But the lactic acid bacteria have not achieved a lasting victory. One theory is that the lactic acid bacteria themselves cannot withstand the harsh effects of the lactic acid they produce. Or do they fall before the attack of some new and unknown microbial champion? Whatever the reason, the lactic acid bacteria begin to quickly disappear. Who will be next to conquer the tank?
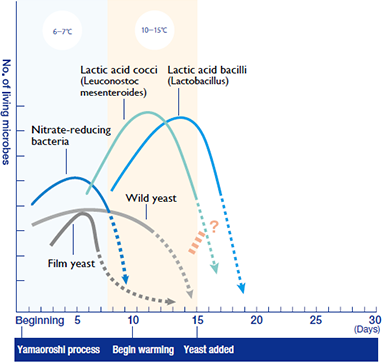
Yeast victorious
The new hegemon is yeast. It doesn’t do well in the presence of nitrous acid and therefore lies low while nitrate-reducing bacteria are present, but with them gone, there is nothing for the yeast to fear. Strong in the presence of lactic acid, the yeast consumes glucose in this acidic environment where other microbes cannot survive. These nutrients fuel growth, making more and more yeast. Eventually the alcohol produced by the yeast kills off the lactic acid bacteria. And so in the end only the powerful yeast, having endured bitter trials and emerged victorious, remains, in a mash with astonishing levels of purity. Yeast has won the day!
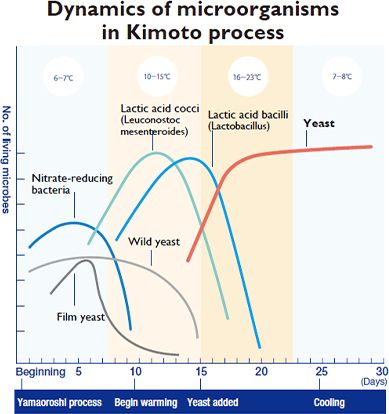
More merits of the kimoto method
Overwhelmingly high purity
Among various ways to make the starter mash, the traditional kimoto method achieves the highest purity of yeast (almost 100%).
The liquid lactic acid added in the initial stage of the sokujomoto method does kill all the bacteria, but it is powerless to keep in check the wild yeast that appears after the acid has broken down and become diluted. In contrast, kimoto brewing has a system in place that culls any wild yeast that may appear.
Astonishing vitality
Kimoto yeast has astonishing vitality. It ferments well at low temperatures, and also enjoys a low death rate at the end of the fermentation process when ordinary yeast is exhausted. Skilled brewers are amazed that it doesn't stop fermenting even when highly-concentrated alcohol is added to make honjozo sake. It is this strength that makes low-temperature long-term fermentation possible. Kimoto yeast is perfect for the manufacture of top-quality sake.
More attractive through maturation
Kimoto sake is characterized by very little deterioration in quality over time. It is thought that because the maturation process is slow, the various components have anti-oxidative properties, making them far less susceptible to deterioration. In other words, by maturation kimoto sake becomes more and more delicious.
Long-lasting fragrance
In order for sake to have a rich bouquet, it is necessary to have both fragrant components and natural preservatives to maintain that fragrance. If the latter are lacking, the fragrance dissipates quickly and then disappears. Kimoto ginjo sake is particularly rich in such preservatives, allowing it to maintain its wonderful aroma.
A lactic acid bacterium
first discovered
at Daishichi Sake Brewery
The research of Prof. Kiyoshi Yoshizawa at Tokyo University of Agriculture revealed that of the five lactic acid bacteria in Daishichi’s kimoto starter mash, one variety possesses a highly unusual enzyme that had never been described before. It seems that this lactic acid bacterium is what gives Daishichi its unique quality, setting it apart from the kimoto mash produced by other companies.
This bacterium's enzyme is an acidic arginase. Although neutral arginases have been discovered before, this is the first arginase known to function in such a low-temperature, acidic environment as the starter mash of sake. Acidic arginase breaks down the amino acid arginine that has an unpleasant bitterness, thus improving the balance of the sake’s flavor components.
The enzyme also works to completely eliminate arginine. This means no harmful ethyl carbamate is produced in Daishichi sake. Let's take a closer look.
- From the early moromi stages, the acidic arginase breaks down the arginine, producing urea.
- The yeast consumes this, leaving behind only trace amounts of urea in the moromi.
- No harmful ethyl carbamate is produced.
The absence of arginine effects a change in how the lactic acid bacteria metabolize amino acids. It has been discovered that the lactic acid bacteria produce aromatic compounds such as high-quality esters. The “Daishichi lactic acid bacterium” performs a host of useful tasks, and is responsible for no drawbacks.














